Lumibird Medical | About us
Lumibird Medical
Lumibird Medical: innovative medical technology benefiting Healthcare professionals and patients around the world
Lumibird Medical is the medical division of the Lumibird Group, an expert in fiber lasers, solid lasers, laser diodes, and medical equipment. Formed from the 2020 merger of three entities – Quantel Medical, Ellex and Optotek – specializing in diagnostic and treatment devices for ophthalmology and other medical fields, our company is recognized for its commitment to innovation and stands out as a world leader in ophthalmic equipment.
Our mission is to improve the comfort of patients worldwide by optimizing the standard of care with innovative diagnostic and treatment solutions dedicated to the medical sector.
Our values
To carry out this mission, we have developed a shared identity structured around four key values. These values are essential to us and guide us everyday in the work we do and the methods we use.
Developing effective, reliable and convenient solutions to meet the needs and requirements of medical professionals and their patients
Mastering knowledge, techniques and environmental conditions related to our sector, monitoring and anticipating trends, and constantly reinventing ourselves by looking towards the future
Ensuring that all stakeholders in our sector – employees, partners, suppliers, customers, and patients – are treated with respect and dignity and fostering a collaborative spirit, communication, and mutual support
Fuelling a desire to always go further and take up new challenges by encouraging curiosity, agility and boldness
A worldwide reputation
An international company with French DNA, Lumibird Medical innovates and extends its operations beyond mainland France to disseminate its expertise and better meet the expectations of healthcare professionals around the world. In addition to our head office in France, we have three production sites, including two abroad, eight international subsidiaries, and two representative offices in Brazil and Thailand. This global presence is supplemented by a network of distributors covering a total of 110 countries.
Decision-making based on a clinical approach
Because physicians everywhere are focused on achieving the very best results for their patients, we at Lumibird Medical decided to involve them in our strategy and work hand-in-hand with them. We therefore devised a collaborative medical ecosystem by creating a Medical Division within our company.
Therefore, as part of clinical studies and the development of new products, the knowledge and medical expertise of all our departments are combined with the clinical experience of our partner physicians, our international KOL network, with a proactive approach resulting from our field operations and our participation in scientific events. This “clinical approach” based on the use of different sources, allows us to be in touch with the needs of healthcare professionals and more easily monitor, or even anticipate, current and future changes.
strong
brands
To remain attuned to the needs of our sector, we offer products sold under three brand names. All three brands are internationally recognized and specialize in diagnostic and treatment equipment for ophthalmology and other medical fields. They offer a comprehensive and tailor-made range of innovative solutions designed to revolutionise healthcare practices.




Quantel Medical
A French brand created in 1993, Quantel Medical specializes in lasers, including retinal lasers for which it is particularly renowned, and medical ultrasound machines dedicated to the diagnosis and treatment of eye diseases.
Since 2018, it has also had a range of interventional imaging systems that meets needs in the areas of general, emergency and musculoskeletal medicine and anaesthesia/resuscitation.

Ellex
Since 1985, the Australian brand Ellex has been developing high-tech laser platforms in the field of ophthalmology.
Recognized as a benchmark on the market of anterior chamber lasers, it also owns “Reflex Technology™”, a light transmission system that ensures optimal illumination during treatment and a precisely positioned aiming beam.

Optotek
Optotek is a Slovenian brand founded in 1990 that is dedicated to medical equipment featuring laser technology, mainly for ophthalmic devices.
Thanks to its ISO-certified production unit based in Ljubljana, it also offers OEM solutions on buoyant markets such as dermatology and ENT surgery.
in 2023
worldwide
A human story,
First of all
Since 1970, when it was created by Mr. Georges Bret, Quantel has been able to adapt to the market to design and manufacture devices for doctors, with the sole aim of improving their practice and facilitating the care of their patients.
Our specialties
Ophthalmology
Our Group manufactures and markets innovative solutions for the diagnosis and treatment of the 4 main causes of blindness – cataracts, glaucoma, age-related macular degeneration (AMD), diabetic retinopathy – and dry eye.
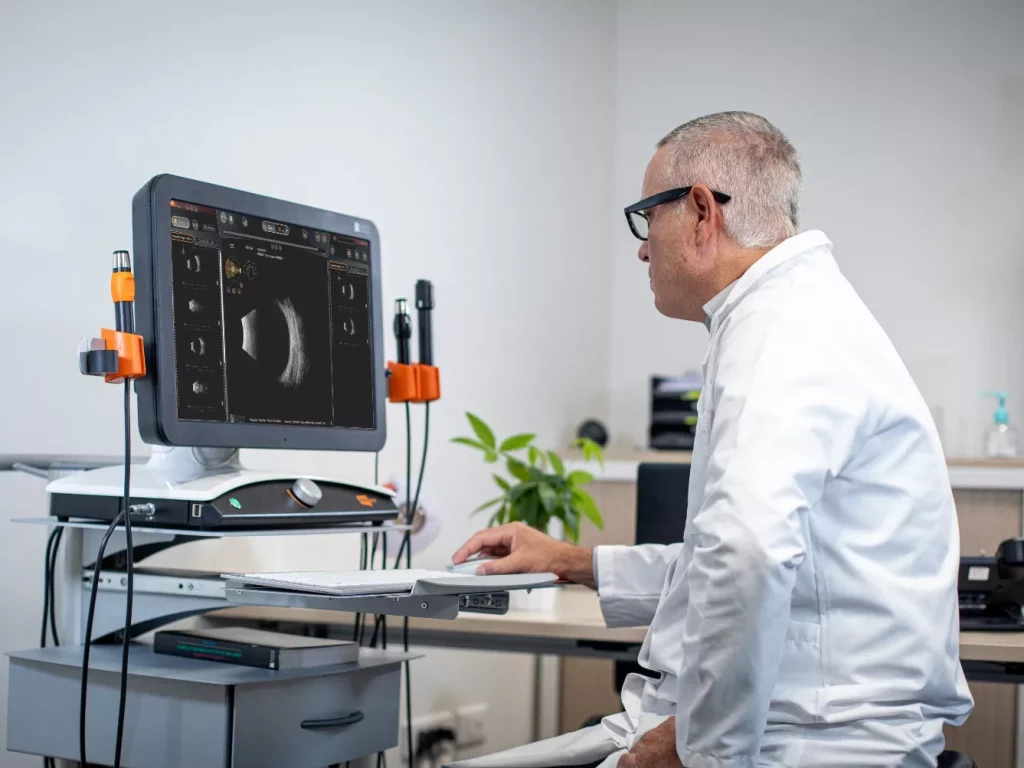
Essential for the screening and prevention of eye diseases, diagnosis depends on the ability of the equipment used to recreate images of extremely complex and tiny structures. We have chosen to address this challenge by developing diagnostic devices using cutting-edge technologies. Thus, while our high-performance ultrasound machines support the diagnosis of cataracts, glaucoma and retinal diseases, our dry eye analyzer offers new possibilities for the contactless diagnosis of one of the main reasons for consulting an ophthalmologist in recent years: dry eye.
Whether it completely cures a disease, stabilizes it or limits its progression to reduced vision or blindness, the goal of treating eye conditions is to improve the comfort of patients. Retinal and anterior chamber lasers drawing from the group’s unique technological expertise are just some of the solutions we have developed for the optimal management of diseases such as cataracts, glaucoma, diabetic retinopathy, and even age-related macular degeneration. We also offer pulsed light (IPL) as an effective treatment for dry eye.

Interventional Imaging
Used to prepare, supplement or even sometimes replace surgery, interventional imaging has become crucial in all fields of medicine. Aware of its importance, we offer a whole range of solutions to meet medical needs.
Ultrasound is a non-irradiating imaging technique used to visualise the body’s various organs. By tapping into technological breakthroughs allowing ultrasound machines to be miniaturised and transported close to patients, we have developed a range of compact, precise and versatile portable devices that perfectly meet the needs of healthcare professionals.
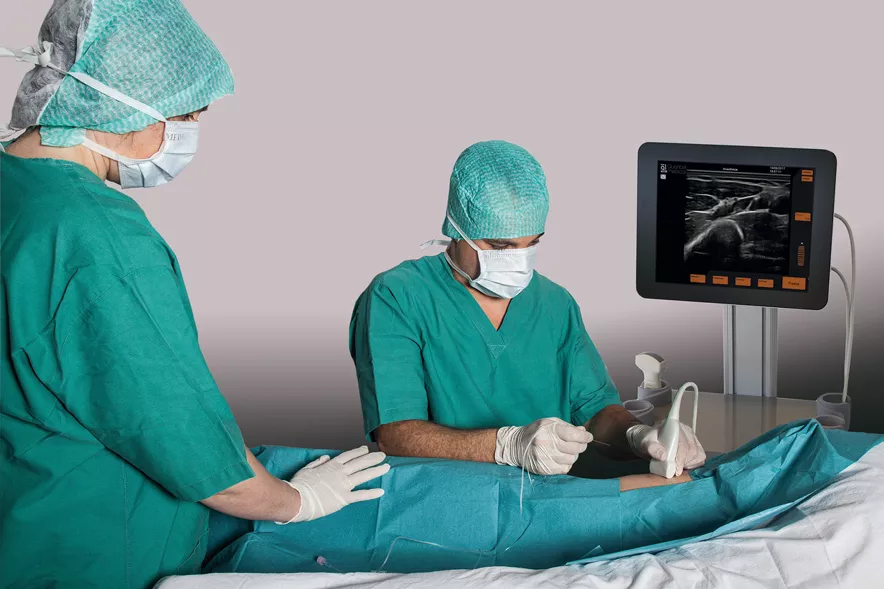

Coupling an ultrasound platform and a percutaneous micro-endoscope in a single device, the EvoTouch+7StarScope brings new functionalities to interventional imaging. With this unique device, procedures that were previously performed blind are now carried out with direct vision.
Management team

With his 30 years of experience, Jean-Marc Gendre‘s renowned expertise in Ophthalmology is not to demonstrate anymore.
Within his strong knowledge in the medical field, he quickly reached a Marketing and Sales Director position at American Medical System. Heading Lumibird Medical, Jean-Marc Gendre gives a steady growth dynamic, enabling the company to strengthen its global leadership position on ophthalmology’s market.
With over 20 years of experience in strategic planning, operational & P&L management, and market development, Ignasi possesses an in-depth knowledge of the global health care industry.
Ignasi has worked and lead public or private companies worldwide and held VP or CEO positions in various companies. In 2021, he founded VHealths Consulting to help MD companies address global challenges.
Ignasi holds bachelor’s in pharmacy and optometry and an MBA.

Within his strong knowledge in the medical field, he quickly reached a Marketing and Sales Director position at American Medical System. Heading Lumibird Medical, Jean-Marc Gendre gives a steady growth dynamic, enabling the company to strengthen its global leadership position on ophthalmology’s market.
Since 2018 he is Lumibird Medical Chief Technology Officer responsible for the technical operational matters, namely the research and industrialisation of Lumibird Medical products.

David Pureur started his career in 1998 within a French company of the optical telecommunications sector. He joined the Quantel group in 2006 in charge of the lasers development in the medical and military fields, before taking the head of the Quantel site in Lannion in 2013.
Since 2018 he is Lumibird Medical Chief Technology Officer responsible for the technical operational matters, namely the research and industrialisation of Lumibird Medical products.
Graduated as an ophthalmologist 32 years ago, in 1993 he obtained his Degree of Doctor of Medicine at the Julius Maximilians University of Würzburg Germany, with a mention in Ocular and Orbital Ultrasound, obtaining the Magna cum Laude qualification. After working as a teacher for several years he won by public competition the habilitation as Principal Professor at the University of San Marcos, the oldest in America.
Mario has opted for a new professional challenge by changing his medical-surgical work to assume the medical direction of Lumibird Medical, with the will to transfer his clinical and teaching experience to his daily work in the company.


After having spent, at the beginning of her career, two years within a refrigerating transportation group as a cash manager, she arrived in the Auvergne and integrated BV international (which became Quantel Medical).
Her position and function have evolved with the structure as the team included less than ten people when she arrived. During 25 years she has contributed to the growing of Quantel Medical – Lumibird Medical through her function as a responsible of the Financial and Management Department.
Bruno started his career as a consultant in quality management. In 2000, he joined EDAP-TMS, a company specialized in urology (prostate cancer and kidney stones) where he was head of department for quality and regulatory affairs. Given the international activities of EDAP-TMS, Bruno has gained a large experience in international environments for both quality and regulatory issues.
He is now at the head of Lumibird Medical’s regulatory and quality affairs since September 2016.


After having spent 8 years within a medical analysis laboratory as electronics engineer in a research office, Patrick Ballandras integrated Lumibird Medical in 2000 to manage the production process of 4 devices.
He is now Lumibird Medical Production Manager: he plays a key role in the organization, planning and monitoring of the production process, while respecting the growing expectations of the company.
Mario has opted for a new professional challenge by changing his medical-surgical work to assume the medical direction of Lumibird Medical, with the will to transfer his clinical and teaching experience to his daily work in the company.
Her position and function have evolved with the structure as the team included less than ten people when she arrived. During 25 years she has contributed to the growing of Quantel Medical - Lumibird Medical through her function as a responsible of the Financial and Management Department.
He is now Lumibird Medical Production Manager: he plays a key role in the organization, planning and monitoring of the production process, while respecting the growing expectations of the company.
He is now at the head of Lumibird Medical’s regulatory and quality affairs since September 2016.
Lumibird,
With 50 years of experience and expertise in 3 key technologies – solid-state lasers, laser diodes and fiber lasers – the group designs, manufactures and markets high performance lasers for the scientific (laboratories and universities), industrial (manufacturing, defense, LiDAR sensors), and medical (ophthalmology) through 5 companies: Quantel Laser, Keopsys, Sensup, Halo Photonics & Lumibird Medical.